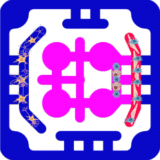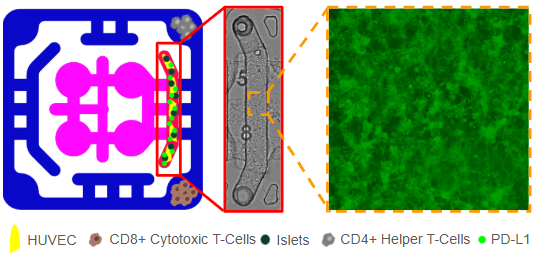
We designed a multiorgan microphysiological plate to model immune rejection of transplanted islets and to test immune-protective strategies. Human pancreatic islets are encapsulated in a fibrin-based hydrogel together with HUVECs. To augment immune checkpoint signaling, the endothelial layer is engineered to present (PD-L1, and additional biomaterial components are introduced as local immunomodulators, such as heparin-mimetic polymers or matrix-anchored cytokines. This arrangement will create a perivascular niche that mimic the transplant microenvironment, supporting islet viability, and establish a local interface for immune regulation.
The encapsulated constructs allow nutrient and insulin exchange while maintaining controlled access to circulating immune cells. In the adjacent outer flow channel, purified CD8⁺ cytotoxic T cells and CD4⁺ helper T cells are introduced under perfusion. These cells are able to sense chemokines and cytokines released by the graft, migrate toward the endothelial interface, and engage checkpoint molecules. This setup allows simultaneous analysis of lymphocyte recruitment, transendothelial migration, and effector responses in the presence or absence of PD-L1 and biomaterial-based interventions.
Functional readouts include T-cell activation states (e.g., CD69/CD25 expression, cytokine secretion, and exhaustion markers), islet function using glucose-stimulated insulin release and viability assays, and endothelial health through barrier integrity and adhesion molecule expression. Soluble biomarkers of inflammation, complement activation, and coagulation are monitored in the flow circuit to capture IBMIR-like events (Instant Blood-Mediated Inflammatory Reaction). Experimental arms will compare PD-L1–expressing versus unmodified endothelial co-cultures, as well as conditions with or without biomaterial immunotherapies, to define the contribution of each protective element to graft survival.
This platform provides a human-relevant model for autoimmune islet transplantation, uniquely integrating therapeutic cells, engineered endothelium, and biomaterial immunomodulation with dynamic immune challenge. It enables systematic dissection of how checkpoint signaling, biomaterial shielding, and vascular support converge to attenuate CD8⁺ and CD4⁺ T-cell–mediated graft rejection, offering a translational tool for optimizing strategies to improve islet transplantation outcomes in Type 1 Diabetes.