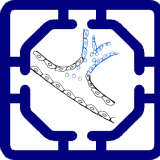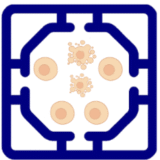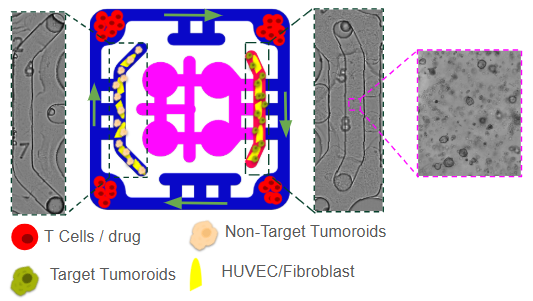
We designed a immuno-oncology model to to evaluate immunotherapy responses under physiologically relevant conditions. The platform incorporates a tumor compartment seeded with three-dimensional spheroids derived from either patient tumors or established cancer lines, embedded in an extracellular matrix-like hydrogel. These spheroids are supported by a surrounding vascular endothelial network that provides nutrient delivery, barrier function, and a conduit for immune cell trafficking. Tumor-associated fibroblasts and macrophages can be included to recreate elements of the tumor immune microenvironment (TME), such as stromal remodeling and immunosuppressive cytokine production.
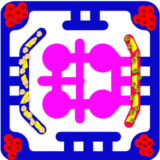
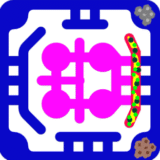
A key feature of this model is the integration of immune cells through the outer perfusion channel. Circulating CD8⁺ cytotoxic T cells and CD4⁺ helper T cells represent autologous tumor-reactive T cells, engineered CAR-T cells, or allogeneic lymphocytes to probe HLA-driven responses. The system also allows inclusion of innate immune components, such as NK cells and monocytes/macrophages, which contribute to tumor clearance and shape adaptive immunity. By co-culturing tumor cells that express immune checkpoint ligands such as PD-L1, this model provides an opportunity to study checkpoint signaling and to test therapeutic blockade with agents like anti–PD-1, anti–PD-L1, or anti–CTLA-4 antibodies. To address the systemic dimension of immunotherapy, the tumor–immune compartment is fluidically linked to secondary organ modules. A liver module, composed of hepatocytes and Kupffer cells, models drug metabolism, cytokine clearance, and immunotoxicity that frequently limit therapy. This multi-compartment design enables simultaneous assessment of both efficacy at the tumor site and off-target safety in peripheral organs.
In the tumor compartment, comprehensive readouts such as tumor cell viability, apoptosis, invasion, and immune infiltration using imaging and single-cell profiling are monitored . Immune cells analyzed for T-cell activation, cytokine secretion (IFN-γ, TNF-α, IL-2), and exhaustion marker expression (PD-1, TIM-3, LAG-3), as well as CAR-T or TCR-engineered T cell persistence and killing efficiency.
This immuno-oncology multiorgan plate is designed for translational relevance. It provides a robust platform to test checkpoint inhibitors, bispecific antibodies, CAR-T therapies, and combination regimens in a human-relevant context that captures both efficacy and toxicity. The ability to model immune cell trafficking, tumor immune evasion, cytokine release, and off-target organ damage within the same system represents a powerful advance over conventional 2D cultures or single-organ chips. Ultimately, this approach has the potential to accelerate immunotherapy development, improve preclinical prediction of clinical outcomes, and reduce reliance on animal models that often fail to recapitulate human immune biology.