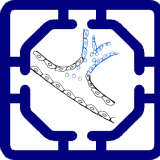
The formation of new blood vessels from existing vasculature, is a central physiological process that can be modeled to recreate vascular niches by embedding human endothelial cells within hydrogel matrices like fibrin or collagen. Under perfusion, these endothelial cells are exposed to physiological shear stress, which stimulates sprouting, lumen formation, and branching similar to in vivo angiogenesis. By integrating multiple organ modules, such as tumor tissues, islets, or liver spheroids, the system captures how different paracrine cues and metabolic signals drive vascular remodeling across organ compartments.
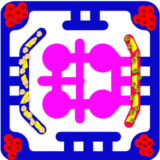
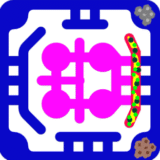
To make the angiogenesis models more physiologically accurate, additional stromal and immune components can be included. Pericytes and smooth muscle cells are added to stabilize vessel sprouts, while fibroblasts contribute extracellular matrix remodeling and secretion of angiogenic factors. Immune cells such as macrophages and neutrophils can be perfused through vascular channels, where they support sprouting by releasing VEGF and matrix-degrading enzymes. Tumor organoids or transplanted islets can be positioned adjacent to the endothelial layer to stimulate angiogenesis through hypoxia-induced VEGF secretion, thereby creating models that mimic tumor-driven neovascularization or graft revascularization.
Structural and functional readouts are studied using confocal microscopy that quantifies sprout length, branching points, and vascular density, or perfusion with fluorescent tracers tests for barrier integrity. Permeability to small molecules or macromolecules, and nutrient or oxygen gradients can be mapped across the angiogenic network. Molecular assays complement these functional measurements by quantifying VEGF, angiopoietins, and HIF-1α secretion, along with endothelial activation markers such as ICAM-1 and VCAM-1.
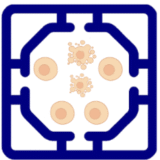
In oncology, tumor compartments are used to drive sprouting, enabling evaluation of anti-angiogenic drugs such as bevacizumab or VEGFR tyrosine kinase inhibitors. In regenerative medicine, ischemia models help test pro-angiogenic therapies or endothelial progenitor cells for tissue repair. In transplantation biology, co-encapsulated islets are linked to vascular modules to study revascularization and survival, a critical step in improving outcomes for Type 1 Diabetes therapies. This enables dynamic, human-relevant studies of vascular biology that capture both local and systemic regulation of angiogenesis.